Covid-19: “It was predictable. To get a vaccine, it will take at least a year, a year and a half” Interview with virologist Gianfranco Pancino
 |
| Giorgio Griziotti 08/04/2020 |
We present a collective interview with Gianfranco Pancino edited by Giorgio Griziotti. Gianfranco Pancino is a virologist and Director of Research Emeritus at INSERM (National Institute of Health and Medical Research).
Tradotto da Fausto Giudice
At the Pasteur Institute in Paris he was a close collaborator of Francoise Barré-Sinoussi, Nobel Prize in Medicine for her research on AIDS and recently appointed by the French government to chair the “Care” (Comité d’analyse, recherche et expertise), a scientific committee for the fight against Covid-19.
I answer a number of questions that have been asked about SARS-CoV-2 and the Covid-19 pandemic. I am a specialist in HIV, which causes AIDS. HIV and SARS have the opposite reproductive strategy. When it infects a host, HIV integrates into its genome and the immune response is unable to eliminate it. The disease is slow and, although the virus is relatively mildly infectious, it has every chance of spreading to other hosts and thus ensuring its own perenniality. SARS-Cov-2 causes an acute infection; it either kills the host in a few days or is eliminated by the immune response. However, the virus is highly contagious and passes quickly to new hosts before it is eliminated. A consolation for today’s times: making a vaccine against an acute infection agent can be less difficult than against a slow virus. Since the immune response can eliminate the virus in recovered patients, it should be possible to induce an effective response with a vaccine. The overwhelming majority of existing effective antiviral vaccines are against acute diseases, polio, measles, mumps, etc.
I will answer, to the best of my knowledge, the questions listed below, without developing controversy, which is moreover justified, about the degradation of health care facilities practiced in recent years by governments of all tendencies, particularly in Italy, France and especially England, or the absolute lack of preparation for the announced threat of viral pandemics. There are other forums and other areas for this, and I hope that we will all be able to reflect on the current drama and draw consequences from it for our way of life and our political systems.
1. How can the virus be passed from animal to human the first time? e.g. by ingestion of meat?
Conspiracy theories about the origin of the Covid-2 pandemic are invading social networks and tabloids. The virus would have been created in a research laboratory, would have been spread by the US military… Why look for such unlikely explanations, when nature can do much better than man in this field. One coronavirus, SARS-CoV (Severe Acute Respiratory Syndrome Virus), had already been responsible for a high mortality epidemic in 2003, and another coronavirus, MERS-CoV (Middle East Respiratory Syndrome Virus), identified in 2012 in Saudi Arabia, and then in many other countries, was responsible for an epidemic in South Korea in 2015. In both cases, there are strong assumptions that the virus’ natural reserve, i.e. the animals carrying these viruses, are bats and that transmission to humans occurred through an intermediate host, the ferret for SARS-CoV and the dromedary for MERS-CoV. The SARS-CoV-2 sequence has a homology of 96% with a Chinese bat coronavirus (Peng Zhou, Nature 2020). It is therefore highly likely that the SARS-CoV-2 virus originates from the bat.
By analogy with the cases mentioned it may have been transmitted to humans by an intermediate host, which could be a wild animal sold in Chinese markets, such as pangolin. In all these cases it is a zoonosis, the transmission of a pathological agent from the animal to humans. Zoonoses that are repeating at an accelerated rate are favoured by human expansion, which destroys the natural habitats of wildlife and puts man in contact with this fauna and its viruses. It would be the proximity and contact with animals that would favour the passage of respiratory viruses such as SARS-CoV, MERS-CoV and SARS-CoV-2 to humans, rather than the consumption of their meat (which in addition is cooked, leading to the destruction of the virus).
2. Incubation times, infection times before the first symptoms and after the end of symptoms?
It is estimated that the incubation period is 2-14 days, with an average of 5.1 days (S. A. Lauer, Annals of Internal Medicine, 2020). A recent study of patients in Wuhan, China, reports that the virus can be released by patients for 20 days (on average) after the onset of symptoms. The shortest period was 8 days and the longest was 37 days (Fei Zhou et al. Lancet 2020). A “cured” patient may therefore continue to be contagious for some time. The virus remains present in serious patients until their death. There are data indicating that the virus could be transmitted not only via the high pathways (droplets of saliva, sneezing, coughing, saliva, etc.) and by contamination of objects, but also by fecal contamination (Charleen Yeo et al., Lancet 2020). The viral RNA was found in the faeces of patients and in the wastewater of two hospitals in Beijing, where patients infected with Covid-19 were hospitalized. A serious problem are people who are infected but remain asymptomatic and therefore not identifiable as virus carriers. These people can be contagious.
3. Is loss of smell/taste such a rare symptom in other diseases that it would indicate a strong likelihood of coronavirus?
Anosmia (loss of sense of smell) and ageusia (loss of taste) can be caused by various causes, including infectious episodes such as a large cold or sinusitis. However, considering various cases reported among patients in China and other countries infected with Covid-2, it is recommended that people who lose their sense of smell and/or taste in the absence of other respiratory diseases, such as allergic rhinitis, acute colds, or chronic sinusitis, warn their doctor to discuss the opportunity of testing for Covid-19 and/or self-isolating.
4. Can influenza vaccination affect the risks of getting sick?
Not that I know of. However, the flu vaccine has no protective capacity against Covid-19.
5. I understand it’s not the swabs that are as scarce as the ability to analyze them. I heard that someone invented a system of swab analysis that lasts only 1 hour: do you think it is possible that you can get a cheap do-it-yourself kit like for pregnancy tests, to see who is immune and who is not? (obviously in the short term because, vaccine or not vaccine, it is the world population that is attacked and therefore before the flock effect in the world years will pass).
The amplification test of the viral RNA from a sample made with the “swab”, is used to know if one is infected, not if s·he is immune. In Italy, as well as in France, there is a shortage of swabs, but also of laboratories equipped to do the test., On March 24, 290 Italian clinicians and researchers have advocated for a radical change in the organization of the tests in an open letter to the Prime Minister and the Governors of the Regions, proposing to connect in a network the Italian research laboratories to carry out the diagnostic tests. Not to accept this proposal on the part of politicians would, in my opinion, be further proof of sloth.
In addition, faster diagnostic tests are entering the market, allowing the amplification of the viral genome within an hour. As for tests based on the detection of antibodies against the virus in plasma, they already exist, but they are only useful to know whether a person has been infected, not for early diagnosis. In fact, these tests only give positive results two weeks after infection. They may be useful for tracking the evolution of the immune response and will certainly be useful for retrospective seroprevalence studies.
6. For the same reasons as above, I think that if all populations were swabbed, we could have a better control of the spread. Am I wrong? If so, why?
To test the entire population in a sweep seems unrealistic and not very useful. It would require millions of swabs and tests, which, apart from representing enormous costs, would be practically unfeasible. Moreover, during the incubation period the test can be negative. I think it makes more sense to swab everyone who has been in contact with virus-positive patients, all healthcare workers, all people in contact with the public, administration employees, police officers, firefighters, supermarket and store clerks, taxi drivers, railway employees, etc. The use of masks should also be extended to all these categories.
7. I would like to better understand the principle of acquiring immunity when you don’t take the disease: Burgio* says that we shouldn’t exaggerate with protections (e.g. disinfect clothes, leave shoes outside, etc.) and don’t stay too closed in the house because we have to form antibodies by taking small doses of virus. But when the virus attacks our cells, doesn’t it multiply rapidly?
Considering that Burgio’s interview is one of the rare serious documents that appeared in the middle of an avalanche of approximations and fake news, I don’t know what Burgio was referring to in that passage. Probably to the fact that a person who comes into contact with an insufficient amount of virus to start an infection or has residual viral products of a virus that is now inactivated (for example after staying on a surface for a long time) can develop an immune response against viral components and therefore have a rapid response if s·he comes into contact with the virus afterwards. However, the virus is very contagious, and can be dangerous. Resuming Burgio’s metaphor about isolation, I would prefer to stay in the mountains waiting for the epidemic to slow down and the antiviral therapies that are being tested are being implemented. Unfortunately this is not possible for the vast majority of the population, so social distancing measures, up to isolation, and hygiene rules are the main defences against the spread of the virus.
8. In the Spanish and Asian flu (partly because I read that the vaccine arrived quite quickly, but I do not remember being vaccinated) the epidemic stopped when it hit 50 or 60% of the population (group immunity) , for Covid-19 if there are no vaccines, when the current wave will lower and we can leave home, is the epidemic not likely to resume as before? So what to do, given that confinement for 1 year until the vaccine arrives will not be possible? The same question in other words: is it true that all the manoeuvres only serve to slow down the epidemic and prevent the collapse of health care, but the only way out of the crisis will be through so-called flock, or herd immunity?
9. Can you explain why it is feared (Burgio/ Imperial college) a second wave more ruinous in autumn as for the Spanish flu? What are the risks?
The flu vaccine was developed in 1938 and then modified at the outbreak of the “Asian” pandemic in 1957-58 to include the predominant virus strain at that time. Its effectiveness was questioned in the scientific literature, and there were two million deaths. Flock/herd immunity, I prefer to say group immunity, is usually achieved through a vaccine. If the vaccine coverage is high enough, the flow of the virus is interrupted. This was the case with smallpox, which was declared extinct. But the coverage must be sufficient, for example in the case of measles it must be more than 93%. By decreasing the frequency of vaccinations, measles reappears, as is happening in our countries. Without vaccine, for a serious disease, such as Covid-19, achieving group immunity allowing the spread of the virus, as initially proposed by Boris Johnson for England, would cause several hundred thousand deaths (at least 250,000 in England, according to epidemiologists estimates), and it is madness. Therefore, for now, it is necessary to be able to contain, or better, to dampen the pandemic. An effective strategy has been put in place by China, which has combined the identification of the sick and infection networks, through testing and social control, and isolation.
The South Korean strategy is different. The mass isolation of the population has been avoided, thanks to rapid and aggressive intervention that has made it possible to identify the first cases and isolate them. By blocking possible new cases of infection from abroad, establishing social separation measures, such as the cancellation of mass events, closing schools and advising people to stay at home, the Korean authorities have managed to flatten the infection curve. However, even if the curve remains flat, the epidemic has not yet ended in South Korea, and we must remain cautious. The intervention was precocious because South Korea, as well as Hong Kong, Taiwan and Japan had prepared for the possibility of a coronavirus epidemic, drawing lessons from the previous SARS outbreak. They had prepared and had adequate diagnostic tests, masks and health care facilities available, and above all precise plans for immediate countermeasures. In addition, all these countries made use of “big data”, the set of personal data of individuals, to track the transmission of the virus and block possible contagion, methods that would be difficult to accept in our democracies.
However, in Western countries, including Italy, there was no forecast or preparation and in the early days the epidemic was underestimated allowing the rapid spread of the virus. Hence the need to resort to late isolation measures at the level of the entire population and the economic slowdown to stop viral transmission. Epidemiologists believe that these restrictive measures can significantly reduce the curve of infection and mortality. The danger is that, as restrictive measures slow down, infection will resume. The model proposed by the epidemiologists at Imperial College in London for the situation in England, of which there is much talk, is as follows:
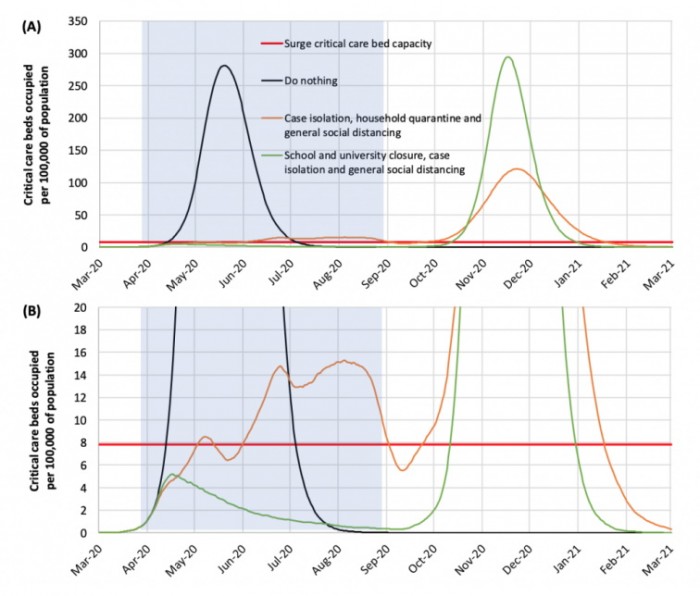
Panel A shows the model. Panel B is a magnification of the bottom of panel A. The grey part on the left corresponds to the first 5 months of the epidemic.
The thick horizontal red line shows the maximum capacity of intensive care beds in British hospitals. The black line shows what would happen if nothing was done (first Boris Johnson option); the green line shows the result of strong restrictive measures (they would be Italian type measures, even if the two situations cannot be superimposed); the orange line represents the effects of a softer policy, which does not imply the isolation of the whole population.
In the case of strong restrictive measures, transmission would be greatly reduced, in the second case of partial restrictive measures, transmission would be contained but not interrupted and the peak of the epidemic would shift to the summer months. The maximum level of supply of intensive care places would be greatly exceeded both by “doing nothing” and, to a lesser extent, by “partial restrictions”. As can be seen on the right of the graph, if the virus were allowed to spread freely, group immunity would be achieved, at the cost of hundreds of thousands of deaths, and a resumption of infection would not be expected. In the absence of vaccination coverage, there is instead the possibility of a new epidemic in autumn-winter, due to an insufficient level of immunity in the population, with a peak similar to the first phase in the case of a rapid suppression of transmission (green line). In short, we would be between Scylla and Charybdis.
Saying that these are models and not safe predictions, how to avoid, or rather parry the possibility of a resumption of transmission? By preparing in time, reinforcing health facilities and screening centres, maintaining capillary surveillance to detect the first signs of a resumption of infection and immediately implementing all the necessary individual containment measures. The course of infection in China and South Korea will provide valuable insights into the future. In the meantime, we will probably be able to count on therapeutic progress, with the identification of effective antivirals against Covid-19. I will not go into drug research, many antiviral molecules are being tested, and the molecules that will prove most effective will be selected in the coming months. Some specific inhibitors of SARS-CoV-2 entry into cells are being investigated. Finally, inhibitors of excessive inflammatory reaction, such as Interleukin-6 (Il-6) antagonists, are also being tested (For more information, see Updated approaches against SARS-CoV-2, Haiou Li et al., Antimicrob. Agents Chemother., 2020).
We won’t have a vaccine, for that we’ll have to wait.
10. Can you explain why Sars and Mers should have made us better prepared? Was it inevitable that sooner or later a highly contagious and dangerous coronavirus would arrive?
There was the SARS and MERS epidemic, there were cases of transmission of avian influenza to humans: zoonoses that had become extinct, at least temporarily, because the viruses had not acquired the ability to transmit effectively from human to human. Since these viruses are capable of frequent mutations and recombinations (the exchange of genome pieces between two or more viruses in the case of co-infection of two CoVs in the same animal or cells), the possibility that a variant capable of transmitting more effectively in humans was only a matter of time. This was the case with SARS-CoV-2 (Saif ur Rehman, Pathogens, 2020). Compared to the first SARS-CoV, the new virus became more effective in binding and penetrating human cells (the cell receptor of SARS CoV-2, ACE2, is expressed on the alveolar cells of the lung) (Walls AC, Cell 2020). Thus much more contagious than the first SARS, although less lethal.
Was it predictable? I leave it to the American CDC (Center for Disease Control), what better source? This is from 2004!
“No one knows if, when, or where person-to-person transmission of SARS-CoV will recur. However, the rapidity of spread of infection and the high levels of morbidity and mortality associated with SARS-CoV call for careful monitoring for the recurrence of transmission and preparations for the rapid implementation of control measures…. Early detection of SARS cases and contacts, plus swift and decisive implementation of containment measures, are therefore essential to prevent transmission.” ((https://www.cdc.gov/sars/surveillance/absence.pdf)
We saw how these recommendations were followed …
11. a) It is true that you cannot start vaccination before the virus is “stable” and that this alone takes 1 year? Why then for influenza (including H5N1) have viruses arrived more quickly?
b) Is it true that the vaccine trial was started at the time of SARS and MERS and irresponsibly stopped because it disappeared?
An RNA virus, such as SARS-CoV-2, is not stable by definition: its reproductive mechanisms fail to accurately copy the viral genome and produce copies with errors. In addition, there are frequent recombinations (see above). Many viral variants are unable to reproduce and are eliminated, some acquire different qualities, such as a greater affinity for the cell receptor, or an ability to replicate more quickly. However, some structures of the virus, which are indispensable for its reproduction, have rather constant patterns and it is on these patterns that vaccine research is concentrated. To design a vaccine it is necessary to know the sequence of the viral genome, to deduce the proteins that make up the virus and to be able to produce the virus on cell cultures.
For influenza, the first vaccine appeared in 1938, 20 years after the Spanish flu pandemic. Since then, virus strains (or sequences for recombinant vaccines) of circulating viruses have been introduced into the vaccine every year. Recommendations for changes are provided to vaccine manufacturers by the WHO (World Health Organisation) 6-8 months in advance, as this is the time needed to produce the vaccine. The vaccine is therefore not perfect because a new variant may appear in the meantime, but it provides some protection, at least from the severity of the disease.
Now, thanks to phenomenal advances in biotechnology it is hoped that we will not have to wait 20 years before having a vaccine against SARS-CoV-2. The virus has been isolated and amplified in cell culture in several laboratories, including the laboratory of the Sacco Hospital in Milan. Immediately after the SARS-CoV-2 sequences were released, many laboratories set in motion to build a vaccine. The search for vaccine candidates is facilitated and accelerated by past research into a vaccine against the first SARS. I don’t know if this research was “irresponsibly” stopped, but no vaccine went into the licensing phase, as the epidemic had stalled.
But now we’re not starting from scratch. Priority is given to the virus protein that allows it to attach itself to the target cell, the spike protein (S). The protein has already been crystallized in its native form, which makes it possible to identify more precisely the antigenic determinants to be introduced in a vaccine (Wrapp D. Science 2020). More than 15 vaccine candidates are currently under study. However, it takes at least one year, one and a half to complete the steps necessary to market a vaccine, from toxicity studies to efficacy studies.
Note
*Ernesto Burgio is an expert in epigenetics and molecular biology. President of the Scientific Committee of the Italian Society of Environmental Medicine (SIMA) and member of the Scientific Council of ECERI (European Cancer and Environment Research Institute) in Brussels. He was interviewed by Giorgio Ferrari on Radio Onda Rossa on 21 March 2020


